
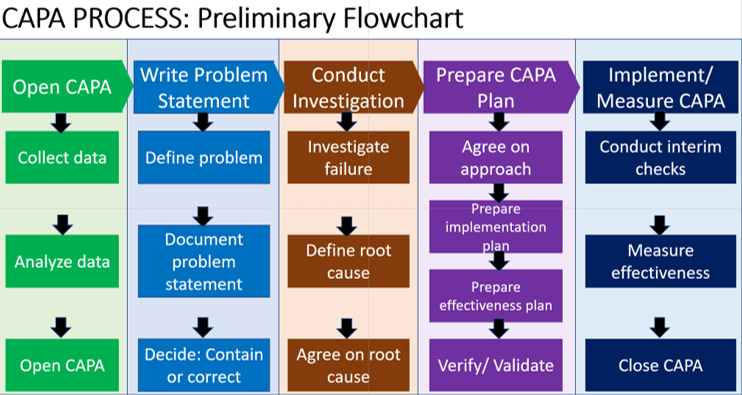

The causes of the problem should be immediately investigated by experienced investigators and never ignored. 3- Investigate and identify the root causes of quality problems It’s important to evaluate the number of CAPAs generated and review that information for quality assurance. Because for the FDA they are always considered as a primary indicator of problems and should be analyzed through the lens of your CAPA system.Īlso, since most investigators judge the quality system by reviewing the CAPA log, it is crucial to record all changes. Keep in mind that it’s important to include both internal and external data sources, and never ignore complaints. 2- Use statistical methodologies to analyze quality data and identify quality problems
In this case, a partnership with a contract manufacturer or company acquisition is recommended. This step might seem obvious, but you would be surprised how many cases have been reported by the FDA as failed on this one.įor small firms, failing to establish a CAPA system often results from a lack of knowledge about FDA and EU requirements, or a product’s questionable regulatory status.įor large firms, the problem can center on contract manufacturers or failing to integrate new sites into the quality system. Here are 5 steps to follow: 1- Implement a CAPA process and document CAPA procedures And recently the FDA has been recommending the implementation of a closed-loop CAPA system in which the CAPA is the tool that drives reports and keeps management informed. And it is evident that the FDA (Food and Drug Administration) investigators look first at the CAPA system during their inspection of the company. Regulatory agencies see the CAPA as the center of all control points including design control, production and process control, records and documents change control, material control, and facility and equipment control. A Corrective and Preventative Action (CAPA) must present a solution to the issue from which the CAPA is generated, or else it is a waste of time and resources.


 0 kommentar(er)
0 kommentar(er)
